Introduction
The aging of the population poses considerable social challenges, as declining health conditions and diseases can lead to disability, dependence and mortality1. Although these conditions are partly reversible such as malnutrition and sarcopenia,2they are associated with a worsening of the underlying disease and increased the risk of mortality.3,4A study reported that malnutrition was associated with 4.4-fold higher mortality risk at 12-year follow-up.3A systematic review and meta-analysis showed a consistent association between sarcopenia and mortality, with a pooled odds ratio of 3.59 and a larger effect in men and women aged 79 years and older.5Therefore, it is important that nutritional risk/malnutrition and sarcopenia are detected early in the elderly.
Due to a series of biological and environmental factors, such as multiple incidence rate, changes in sensation and body composition, loss of appetite, oral health decline, malabsorption, multiple drugs, financial difficulties and social isolation, the risk of malnutrition and micronutrient deficiency increases among older adults.6Malnutrition in elderly inpatients is associated with decreased muscle quality, impaired function, decreased quality of life, increased use of medical resources, prolonged hospital stay and increased mortality.2,7,8Nutrition assessment has been included in the comprehensive geriatric assessment (CGA), which is the core and important part of the comprehensive management of the elderly.9The nutritional risk screening 2002 (NRS 2002) scale is a popular nutritional risk screening tool among hospitalized patients. It was first introduced by Kondrup et al and validated by the European Society for Clinical Nutrition and Metabolism.10However, subjects have no choice but to spend a lot of time answering questionnaires.
Sarcopenia is characterized by progressive and systemic loss of skeletal muscle mass (SMM) and strength, which may lead to physical disability, poor quality of life and other adverse consequences.11Sarcopenia is now recognized as a muscle disease by World Health Organization. The 10th Revision of the international classification of diseases (ICD-10-CM) diagnostic code is M62.84.12Up-to-date, most international consensus holds that the diagnosis of Sarcopenia should include low muscle mass and decreased muscle function. Low muscle mass is usually measured by magnetic resonance imaging (MRI), computed tomography (CT), dual energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA).13However, these methods are time-consuming, inconvenient, reliable, and some of them are expensive and may cause radiation exposure. Muscle dysfunction is usually estimated by grip strength and gait speed, which also requires specific equipment or experienced trainers.
Nutritional risk and sarcopenia cause serious adverse consequences including death. It is necessary to detect and intervene early. Low serum creatinine has been not only used as a substitute for low muscle mass but also associated with adverse outcomes.14,15Cystatin C is a small protein derived from all nucleated cells. Its production is relatively stable and less affected by SMM.16Therefore, Kashani et al, recently developed a new sarcopenia index (SI, serum creatinine [mg/dl]/cystatin C [mg/l]) × 100) was used to estimate SMM.17A research showed that SI was a fair measure of muscle mass and can moderately predict in-hospital mortality in intensive care unit (ICU) patients with a stable renal function (excluding evolving or recovering acute kidney injury and need for renal replacement therapy).18Unlike other complex tools, SI was only based on serum creatinine and cystatin C, which are objective and routine tests in the elderly. If SI could accurately estimate sarcopenia in the elderly, it would be of great value to simplify the diagnosis procedure of sarcopenia.19Because of the convenience of detection, SI is a hot spot in various research fields. In the cardiovascular field, SI, surrogate for the degree of SMM, could be used as a predictor of adverse outcomes in patients undergoing transcatheter aortic valve replacement.20SI also could predict chemotherapy-induced undesirable effects and mortality in older People with stage III or IV non-small cell lung cancer.21
In our study, we analyzed the data of a prospective study to investigate the association of SI with mortality, nutritional risk/malnutrition and sarcopenia among hospitalized older Chinese adults.
Methods
We analyzed the data of a prospective study that a total of 1234 hospitalized patients admitted to the Department of Geriatrics at Shanghai Jiaotong University Affiliated Sixth People’s Hospital between July 2017 and April 2018. We excluded patients with cachexia (referent to the clinical history), hemodialysis, peritoneal dialysis, acute renal insufficiency or severe diseases (including acute myocardial infarction, acute heart failure, acute stroke, severe infection) that made them fail to communicate with the interviewers. We also exclude patients with no record of serum creatinine and cystatin C measured upon admission. Finally, 758 hospitalized participants were included in the analysis (Supplemental Figure 1). The study was approved by the Ethics Committee of the Shanghai Jiaotong University Affiliated Sixth People’s Hospital [approval number, 2016–141-(1)]. Written informed consent was obtained from all participants and in accordance with the principle of the Helsinki Declaration. Written informed consent was obtained from each participant.
Detailed information about medical history and lifestyle, including smoking and drinking status, was obtained using questionnaires and confirmed through examination of medical records by trained physicians. Current smoking status was defined as yes if the subject smoked at least one cigarette per day or seven cigarettes per week in the past 6 months. Current drinking status was defined as yes if the subject consumed alcohol at least once a week in the past 6 months. Blood pressure (BP) was measured at the non-dominant arm in a seated position after a 5-min rest using an automated electronic device (OMRON Model HEM-752 FUZZY’ Omron Co., Dalian, China). Three measurements were taken one minute apart, and the average of the three measurements was used in the analysis. The diagnostic criteria of overweight and central obesity, type 2 diabetes (T2D) and hypertension were defined according to the Adult Treatment Panel III guidelines.20Overweight was defined as body mass index (BMI)≥25kg/m2. Coronary heart disease was identified if patients had a myocardial ischaemic history or electrocardiographic typical ischaemia pattern. Cerebral infarction was confirmed as a history of ischaemia attack confirmed by cerebral CT or MRI scan. Estimated glomerular filtration (eGFR) was calculated using MDRD equation: 186 × (Creatinine/88.4)−1.154× (Age)−0.203× (0.742 if female).22Where unit of eGFR = mL/min/ 1.73 m2, serum creatinine = μmol/L and age = years. Renal dysfunction (RD) and non-RD were defined as eGFR <90 mL/min/1.73 m2and eGFR ≥90 mL/min/ 1.73 m2, respectively.
Body height, weight, and waist circumference (WC) were measured by experienced physicians. Height and weight were recorded to the nearest 0.1 cm and 0.1 kg, respectively, while participants were wearing light indoor clothing without shoes. BMI (kg/m2) was calculated as weight in kilograms divided by height in square metres. WC was measured to the nearest 0.1 cm with participants in the standing position. The greatest circumference of the lower right leg was measured in the standing position as calf circumference (CC).
The patient took the seat, bends the knee and bends the hip 90°, and the 2 ft are naturally placed on the ground; the shoulders remain adducted, the upper arm is flat with the chest, the forearm is neutral, and the elbow is bent to 90°. The handle of the dynamometer was adjusted if required - the base should rest on the first metacarpal (heel of palm), while the handle should rest on the middle of the four fingers. The maximum grip strength of the dominant hand (WCS-100 electronic vibrometer, China) was measured three times, and each time after the measurement, the rest was taken for 1 min. The result was taken as the maximum value of three times.
NRS 2002 was used to determine nutritional risk. The total NRS 2002 score indicates whether the patient is nutritional risk/malnutrition (score ≥ 3) or normal (score < 3).23A multidisciplinary nutrition support team evaluated the nutritional status of each patient. All patients underwent nutritional status assessment in the first 24 h of hospital stay.
The Asian Working Group for Sarcopenia (AWGS) 2019 algorithm24revised the consensus definition of sarcopenia recently: Criterion 1: Low muscle strength; Criterion 2: Low muscle quantity or quality; Criterion 3: Low physical performance. Probable sarcopenia is identified by Criterion 1. The diagnosis is confirmed by additional documentation of Criterion 2. If Criteria 1, 2, and 3 are all met, sarcopenia is considered severe. In this study, the participants did not accept physical performance tests. We only used low muscle strength and low muscle quantity as confirmed sarcopenia. According to AWGS 2019 algorithm recommended cutoffs, HGS < 28 kg in men or < 18 kg in women were considered as low muscle strength. CC lower than 34 cm in men or 33 cm in women were considered as low muscle quantity.
All patients fasted overnight before blood samples were collected. The measurements of creatinine, cystatin C, fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c) were serum albumin were assessed using turbidimetric immunoassay (Hitachi, Tokyo, Japan). Creatinine were measured by an enzymatic method with an automatic biochemical analyzer (7600–020, Hitachi, Tokyo, Japan). The normal reference value is Haemoglobin levels were measured using a standard cyanmethemoglobin method. Haemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography using Bio-Rad D-10 (Bio-Rad, Inc. USA). TyG index was calculated as ln (fasting TGs [mg/dL] × fasting glucose [mg/dL]/2). SI was calculated as serum creatinine/serum cystatin C × 100.
All the participants accepted follow-up in the geriatric clinic of Shanghai Jiaotong University Affiliated Sixth People’s Hospital. The deadline for the follow-up was July 30, 2018. The median of the follow-up period was 212 days. All deaths occurring between study entry and deadline were included. Due to the participants accepting healthcare at Shanghai Jiaotong University Affiliated Sixth People’s Hospital, there were no missing follow-ups.
SAS version 9.1 (SAS Institute, Cary, NC) was used for database management and statistical analysis. Data are presented as the means ± standard deviation (SD) for continuous variables or numbers (percentages) for categorical variables. For continuous variables, a one-way ANOVA was used to detect differences across groups, and Fisher’s Least Significant Difference (LSD) post hoc analysis was used to determine the difference between every two groups. For categorical variables, the chi-squared test was used to detect the difference across groups. When a significant difference was identified across groups, column proportions tests (z-tests) with Bonferroni correction were performed to determine the difference between every two groups. The association between SI and other variables was evaluated with Pearson or Spearman correlation analysis. The differences between the four groups were evaluated with the ANOVA test. Categorical variables were presented as frequency percentage, and intergroup comparisons were analyzed using the chi-square test. Univariate and multivariate Cox proportional-hazard models were applied to calculate the unadjusted and adjusted hazard ratio (HR) and 95% confidence interval (CI) of the SI for mortality. The univariate and multiple stepwise Logistic regression analysis were adopted to identify the influencing factors nutritional risk/malnutrition and sarcopenia. The SI was treated as either a continuous variable (per 1-SD= 22.1) or a categorical variable (using the quartile cut-off points: Q1<56.6, Q2 56.6–72.3, Q3 72.3–85.8, Q4>85.8). Age, sex, and other confounders which were significantly different between the quartile groups of SI were adjusted for in the Cox proportional-hazard models and multiple stepwise Logistic models.
Results
A total of 758 participants agreed to participate in this study (589 men and 169 women; mean age: 85.6±6.1 years). The median of the follow-up period was 212 days and 112 patients died. The baseline characteristics of participants according to the quartiles of SI were shown in Table 1. The proportion of male was higher in the Q4 group than in the other three groups. The mean age of the participants in the Q4 group was significantly lower than the other three groups. The eGFR and prevalence of T2D, CHD and COPD were significantly higher in the Q1 group than in the other three groups, whereas the albumin, haemoglobin, BMI, WC, CC, HGS and the prevalence of RD were the lowest in the Q1 group. SBP was higher in the Q3 group than in the other three groups.
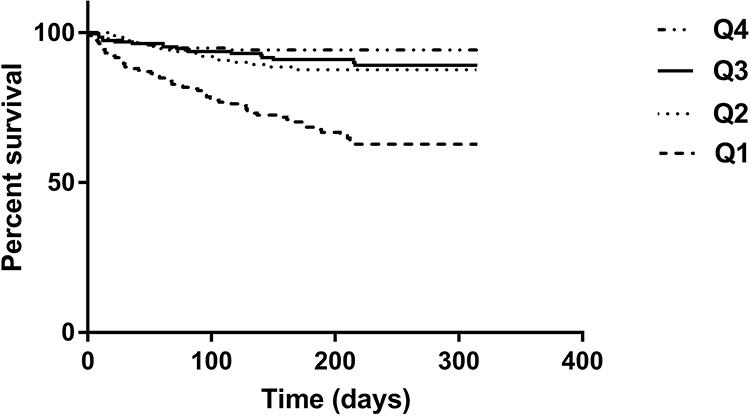
Table 1 Baseline Characteristics of Participants According to the Quartiles of Sarcopenia Index |
Mortality and the prevalence of nutritional risk/malnutrition and sarcopenia were showed in Figure 1A-C. During 1-year follow-up, a total of 112 participants (14.8%) died. The mortality according to the quartiles of the SI was 32.4%, 11.2%, 9.6% and 5.8% in the Q1, Q2, Q3, and Q4 groups, respectively (P for trend <0.001). The prevalence of nutritional risk/malnutrition and sarcopenia was all highest in the Q1 group (66.5% and 92.7%) and gradually decreased with the increase of SI (all P for trend <0.001).
Figure 1 (A) The prevalence was 32.4%, 11.2%, 9.6% and 5.8% for mortality from the lowest to the highest sarcopenia index quartile, respectively (P for trend < 0.001). A significant decrease of mortality was observed in Q2, Q3 and Q4 group compared to the Q1 group. (B) The prevalence was 66.5%, 43.1%, 23.9% and 15.2% for nutritional risk/malnutrition from the lowest to the highest sarcopenia index quartile, respectively (P for trend < 0.001). A significant decrease of prevalence of nutritional risk/malnutrition was observed in Q2, Q3 and Q4 group compared to the Q1 group. A significant decrease of prevalence of nutritional risk/malnutrition was also observed in Q3 and Q4 group compared to the Q2 group. (C) The prevalence was 92.7%, 83.0%, 76.6% and 59.7% for sarcopenia from the lowest to the highest sarcopenia index quartile, respectively (P for trend < 0.001). A significant decrease of prevalence of sarcopenia was observed in Q2, Q3 and Q4 group compared to the Q1 group. A significant increase of prevalence of sarcopenia was observed in Q2 and Q3 compared to the Q4 group. Q stands for sarcopenia index: Q1 is the lowest quartile and Q4 is the highest quartile. Cutoffs for sarcopenia index are Q1<56.6, Q2 56.6–72.3, Q3 72.3–85.8, Q4>85.8.aSignificantly different from the Q1 group.bSignificantly different from the Q2 group.cSignificantly different from the Q3 group.dSignificantly different from the Q4 group. |
The high SI was positively correlated with HDL-c (r=0.10, P=0.014), albumin (r=0.32, P<0.001), hemoglobin (r=0.24, P <0.001), BMI (r=0.12, P=0.001), WC (r=0.08, P=0.046), CC (r=0.45, P<0.001) and HGS (r=0.52, P <0.001) (Table 2). The high SI was negatively correlated with age (r=−0.30, P<0.001), male (r=−0.31, P<0.001), FPG (r=−0.11, P=0.005), TG (r=−0.09, P=0.020) and TyG (r=−0.11, P=0.007) (Table 2).
Table 2 Correlation Risk Factors with Sarcopenia Index |
When the SI was treated as a continuous variable (per 1-SD), a higher SI was independently associated with a lower risk of 1-year all-cause mortality after adjusting for potential confounders (HR per 1-SD= 0.61, 95% CI: 0.47–0.79) (Table 3).
Table 3 Association Between Sarcopenia Index and Mortality (1-Year Follow-Up) According to Cox Regression Models Adjusted for Potential Confounders |
We also evaluated the association between different quartiles of SI and 1-year all-cause mortality. After adjusting for potential confounders, the Q1 group was associated with 1-year all-cause mortality (HR=2.39, 95% CI: 1.11–5.18) when using the Q4 group as the reference. The Q2 and Q3 groups were not significantly associated with 1 year all-cause mortality (HR= 0.95, 95% CI: 0.42–2.14 and HR=1.22, 95% CI: 0.57–2.63) (Table 3).
Figure 2 shows the survival curves of the participants in different quartile groups according to the SI. The Log rank test indicated that the survival curves of the participants in different quartile groups were significantly different (P<0.001).
Figure 2 Survival curves of the study population according to the quartiles of sarcopenia index. Log rank test χ2 = 68.47, P < 0.001. |
When the SI was treated as a continuous variable (per 1-SD), a higher SI was independently associated with a lower risk of nutritional risk/malnutrition after adjusting for potential confounders (OR per 1-SD= 0.38, 95% CI: 0.29–0.49) (Table 4).
Table 4 Association Between Sarcopenia Index and Nutritional Risk/Malnutrition According to Logistic Regression Models Adjusted for Potential Confounders |
We also evaluated the association between different quartiles of SI and nutritional risk/malnutrition. After adjusting for potential confounders, the Q1 and Q2 groups were all associated with nutritional risk/malnutrition (OR=10.60, 95% CI: 5.65–19.89; OR=3.88, 95% CI: 2.19–6.87) when using the Q4 group as the reference. However, Q3 group was not significantly associated with nutritional risk/malnutrition (OR=1.71, 95% CI: 0.98–2.98) (Table 4).
When the SI was treated as a continuous variable (per 1-SD), a higher SI was independently associated with a lower risk of sarcopenia after adjusting for potential confounders (OR per 1-SD=0.58, 95% CI: 0.45–0.74) (Table 5).
Table 5 Association Between Sarcopenia Index and Sarcopenia According to Logistic Regression Models Adjusted for Potential Confounders |
We also evaluated the association between different quartiles of SI and sarcopenia. After adjusting for potential confounders, the Q1, Q2 and Q3 groups were all associated with sarcopenia (OR=4.42, 95% CI: 1.98–9.85; OR=2.42, 95% CI: 1.30–4.51; OR=1.99, 95% CI: 1.17–3.36) when using the Q4 group as the reference (Table 5).
Discussion
Due to the defects of current diagnostic methods for sarcopenia, SI is an attractive method to verify a specific biomarker as an alternative marker for sarcopenia.25As a new member of this field, SI has low cost, high accessibility and repeatability.26Therefore, SI was studied in different populations such as cancer21,27chronic liver diseases,28severe patients,29peritoneal dialysis patients,30older people with normal kidney function31and so on. However, in this follow-up study of 758 hospitalized participants, we firstly demonstrated the association between the SI and mortality, nutritional risk/malnutrition and sarcopenia in the elderly including the patients with RD.
Only few previous studies addressed the association between the SI and mortality. In two retrospective studies conducted in adult ICU patients, a lower SI was associated with a significantly higher risk of mortality among adult ICU patients.17,18Another study demonstrated that the SI was associated with long-term mortality among hospitalized older patients in a normal kidney function population19.Therefore, more prospective studies are needed before strong conclusions can be drawn. Our finding was in line with these findings according to multivariate Cox proportional-hazard models after adjusting for potential confounders.
Our study confirmed that the SI was positively correlated with nutritional status indexes such as albumin, hemoglobin, BMI and WC. More importantly, our study confirmed that the SI could predict nutritional risk/malnutrition in older patients after adjusting for potential confounders for the first time.
Sarcopenia and nutritional risk/malnutrition often occur in the elderly. A study showed that the SI might not serve as biomarkers of either low muscle mass or sarcopenia in urban community-dwelling older people with normal kidney function.31However, we showed that the SI was positively correlated with indexes of muscle mass and muscle strength such as CC and HGS. We also demonstrated that the SI could predict sarcopenia after adjusting for potential confounders including RD in the elderly. Therefore, the association between the SI and sarcopenia is still controversial. More prospective studies are needed to verify the value of SI in different populations.
Serum creatinine is mainly affected by physiological and clinical conditions that affect muscle quality.32Cystatin C levels can provide a more useful estimate of renal function in patients with reduced muscle mass because it is free to filter glomerular low molecular weight protein with stable productivity.33Because serum cystatin C values depend on glomerular filtration volume, they are not affected by early renal insufficiency, unlike creatinine. Low serum creatinine/cystatin C ratio was associated with low muscle mass in various conditions.33,34These studies have proved that muscle markers are reliable. These results suggest that the creatinine/cystatin C ratio eliminates the effect of potential differences in renal function. Malnutrition is accompanied by loss of muscle mass and muscle function and exaggerates the observed loss of fat-free mass in elderly persons, reducing metabolic reserve and insulin sensitivity.35TyG is a new and reliable surrogate marker of insulin resistant and is calculated using TG and FPG,36which was related of the SI in our study. In patients with low muscle mass, decreased numbers of muscle cells and decrease endocrine function could contribute to poor clinical results.37,38Some factors including preexisting chronic comorbidities, sarcopenia, nutritional risk/malnutrition explain why the mortality is highest in Q1 group in our study.
This study had some strengths. First, all patients had well compliance and they were average of 85 years, who are usually excluded from many studies. Second, this study included a large sample which could better reflect the status of elderly patients than previous studies. Finally, we recorded the patient’s diseases in detail.
This study also had some limitations. First, the definition of sarcopenia is weak. Gold standard of sarcopenia is psoas area muscle measurement by CT or MRI. Correlation between Psoas muscle area and SI would be ideal. We did not design physical performance tests in the beginning of the study because of patients’ age and inconvenient movement. Second, when any disease was found, all patients were intervened. We did not include the effects of drugs or other interventions in the study. Therefore, in order to decrease the effects of interventions, we followed up about 1 year. Third, due to the low proportion of female subjects in this study, we were unable to estimate the potential gender impact. Finally, this is a single-centre study of inpatients in geriatric wards. All the participants in the study were Chinese so that the results may not be applicable to other ethnic groups.
Conclusion
The SI based on serum cystatin C and creatinine is associated with long-term mortality, nutritional risk/malnutrition and sarcopenia in a study population of hospitalized older Chinese patients. The SI helps us identify people who are at a high risk of adverse outcomes. Future studies should be conducted to prospectively validate the SI and explore clinical applications for nutrition and sarcopenia therapy.
